
PRODUCTS
PRODUCTS
Product Details
Ethylene glycol, commonly known as glycol, also known as 1,2-ethylene glycol or ethylene glycol, is the simplest aliphatic diol with a chemical formula of C2H6O2 and a molecular weight of 62.07. It is typically a colorless, transparent, viscous liquid with a sweet taste and is hygroscopic. It is slightly soluble in ether and practically insoluble in benzene and its homologues, chlorinated hydrocarbons, and petroleum ether. It is miscible with water, lower aliphatic alcohols, glycerol, acetic acid, acetone, and other similar ketones, aldehydes, and pyridine. It can oxidize to acids when exposed to strong oxidants. When heated in the presence of a catalyst (manganese dioxide, aluminum oxide, zinc oxide, or sulfuric acid), it undergoes intramolecular or intermolecular dehydration to form acetaldehyde, ethylene glycol acetal, or dioxane. It can react with alkali or alkaline earth metals to form alkoxides.
Ethylene glycol can be obtained by hydrolysis of chloroethanol in an alkaline medium or by catalytic or direct hydration of ethylene oxide. It is primarily used in the production of polyethylene terephthalate (the raw material for polyester fibers and polyester plastics) and is also used in the synthesis of pharmaceuticals, pesticides, resins, surfactants, softeners, and explosives. It can also be used as a reagent, antifreeze, desiccant, and as a convective heat transfer medium in automobiles and liquid-cooled computers.
Contact or inhalation of ethylene glycol can cause irritation to the skin, nose, and throat. Accidental ingestion can result in mild symptoms similar to those of drunkenness, while severe cases can manifest as paroxysmal confusion, lethargy, coma, convulsions, incontinence, and cerebral edema. Seizures can be tonic-clonic, and patients may experience nystagmus and optic disc edema. Patients who enter a coma may experience hypotension, tachycardia, tachypnea, and cyanosis. Severe cases can develop pulmonary edema, cardiomegaly, and congestive heart failure.
PRODUCT PROPERTIES
Appearance: Colorless, transparent, viscous liquid
Melting Point: -13°C (lit.)
Molecular Weight: 62.068
Boiling Point: 195-198°C
Glass Transition Temperature: -120°C
Vapor Pressure: 0.08 mmHg (20°C)
Density: 1.113 g/mL at 25°C (lit.)
Refractive Index: 1.4472 (λ: 589.3 nm; Temp: 20°C)
Viscosity: 25.66 mPa.s (16°C)
Heat of Combustion: 1180.26 kJ/mol
Autoignition Point: 418°C
Critical Temperature: 372°C
Critical Pressure: 7699 kPa
Critical Molar Volume: 186 C³/mol
Eccentricity Factor: 0.27
Surface Tension: 46.49 mN/m (20°C)
CAS Database: 107-21-1
PRODUCT APPLICATIONS
Mainly used in the production of polyester, terylene, polyester resins, desiccant, plasticizer, surfactant, synthetic fibers, cosmetics, and explosives.
It is also used as a solvent for dyes and inks, as a compound for engine antifreeze, as a gas dehydrator, in the manufacture of resins, and as a wetting agent for cellophane, fiber, leather, and adhesives.
It can be used to produce synthetic resin PET, fiber-grade PET (i.e., polyester fiber), and bottle flake-grade PET for mineral water bottles. It can also be used to produce alkyd resins and glyoxal, and is also used as an antifreeze.
In addition to its use as an automotive antifreeze, it is also used for industrial refrigeration transport, generally referred to as a coolant. It can also be used as a condensing agent, similar to water.
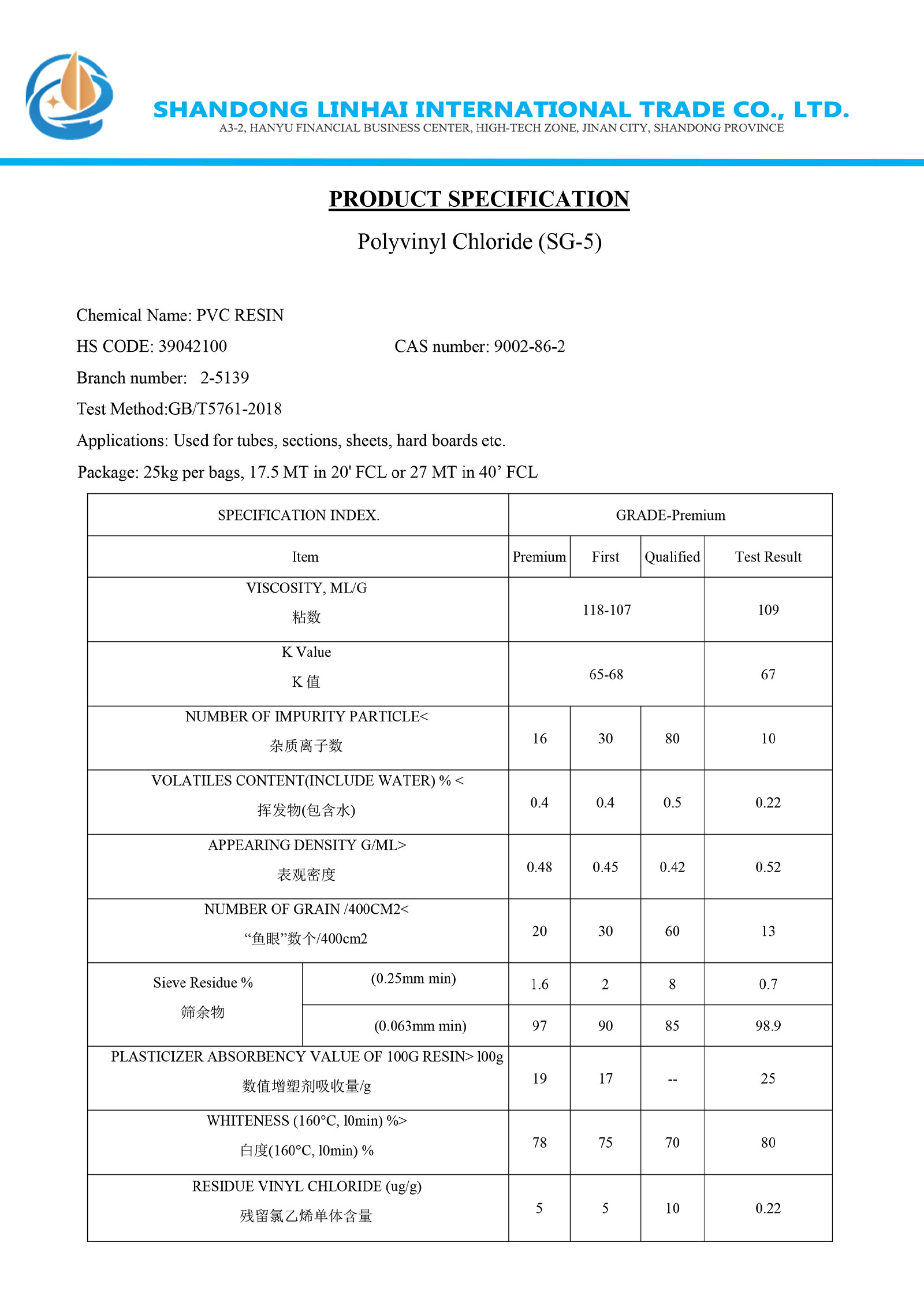
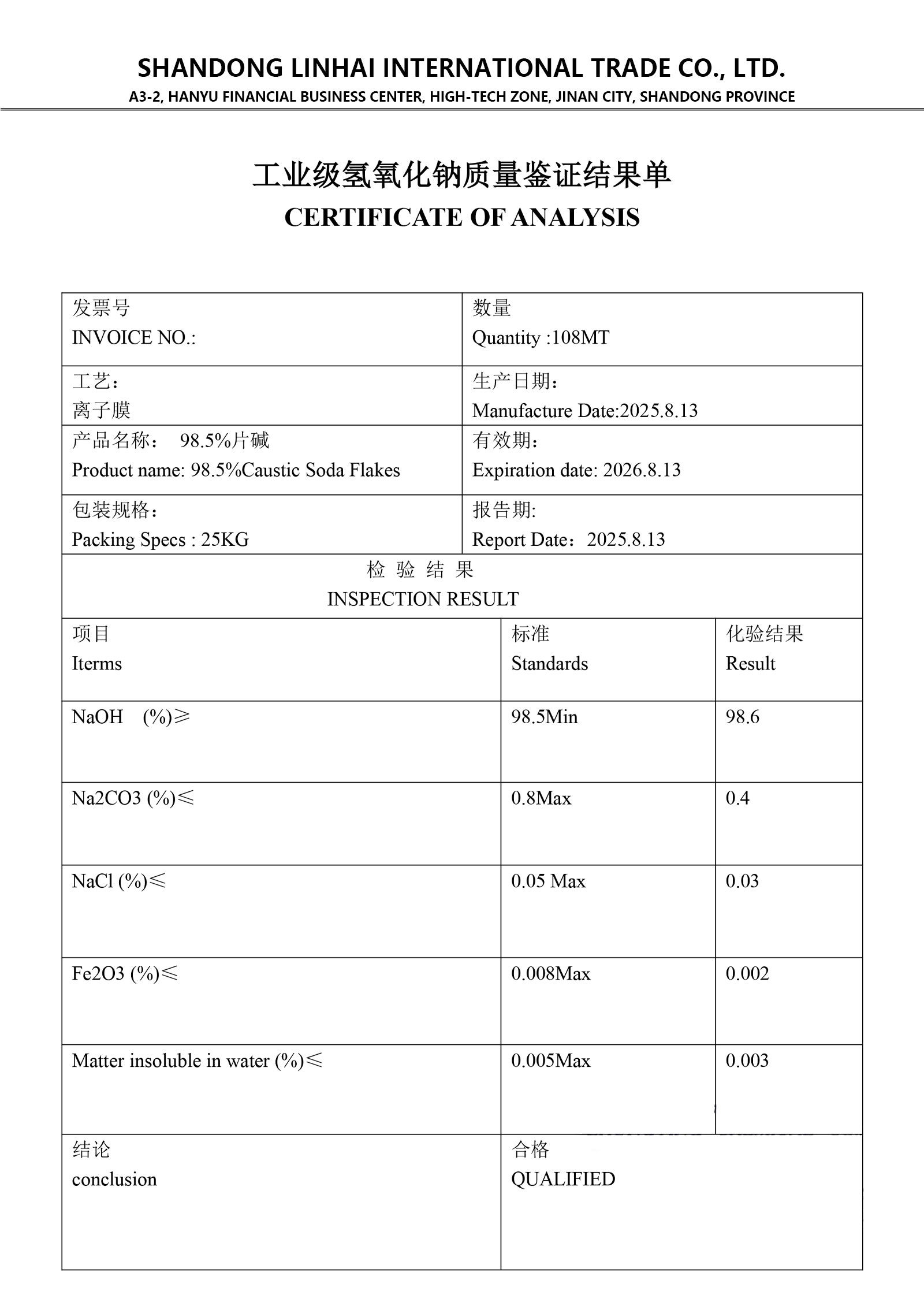
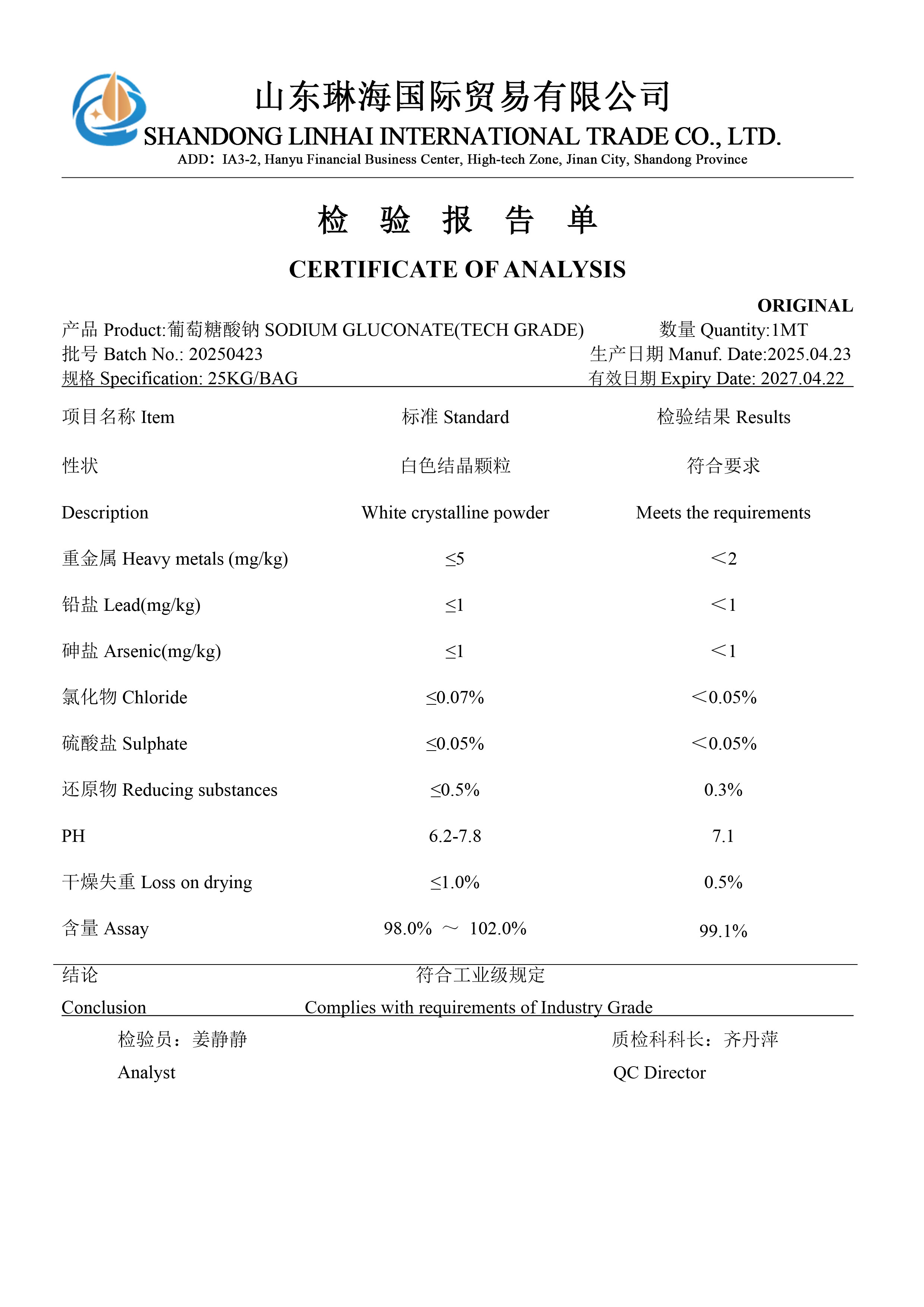
Material list certificate

Factory & Shipping

Company Introduction
We possess advanced production equipment and a technical team with extensive industry experience and expertise. We have established a strict production management system and are certified to ISO9001 quality management systems.
We prioritize product safety, environmental protection, and social responsibility. Our core products comply with food contact material standards and regulations issued by major countries and regions worldwide, including those issued by the National Health Commission of China, the U.S. Food and Drug Administration, the Ministry of Health, Labour, and Welfare of Japan, and the European Commission. These products meet the increasingly stringent safety and environmental requirements of food contact materials and medical supplies.

Online Consultation
Related Suggestion
Get in Touch
*We respect your confidentiality and all information are protected.







